A 1.5 million dollar grant has been given to a peanut allergy immunology
study for Phase 3. Research will continue the method used. It is also hoped
that this method can be introduced and used in Europe and the United
States.
Company to Begin Phase 3 Trials of New Peanut Allergy OIT Candidate with $1.5 Million Innovation Award

Cambridge Allergy Ltd (Camallergy) — the Cambridge UK-based biopharmaceutical company — announced today that it had received a £1.1 million ($1.5 million) Innovate UK Biomedical Catalyst Award from the United Kingdom’s research and innovation agency.
The grant will be used to accelerate phase 3 trials of CA002 — the company’s peanut allergy oral immunotherapy (OIT) candidate — slated to begin this quarter.
Said Dr Sarah Oakley-Mudge, Director of Clinical Development:
This innovation award is further testament to Camallergy’s leadership and capabilities in the field of peanut allergy immunotherapy. With the only cGMP-certified manufacturing facility in Europe dedicated to producing peanut allergy immunotherapy for human use we believe we are well positioned to utilize this award to accelerate the development of CA002 into Phase 3 trials and towards commercialization.
Click to visit sponsor
CA002 is a peanut protein formulation delivered in pre-measured capsules that are split apart and spread on food.
Camallergy’s website claims:
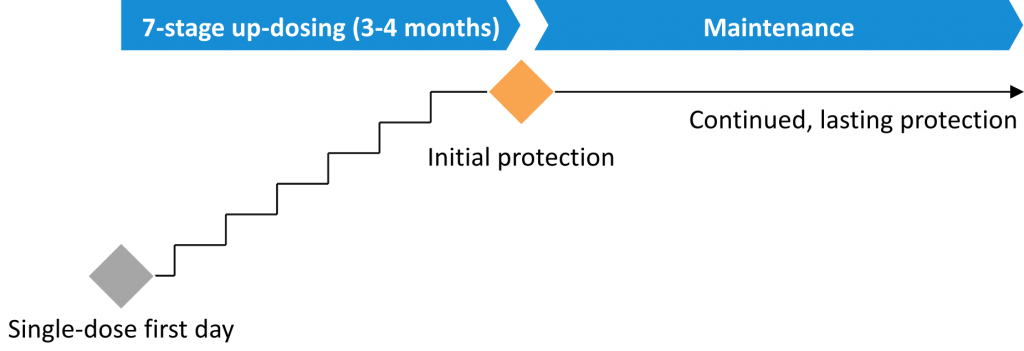
The short, first-day appointment requires only a single treatment dose, avoiding the long, stressful and unpredictable first day of traditional ‘rush’ up-dosing therapies. This is typically followed by only six further brief visits to the allergy office. The treatment is designed for low burden to the patient and physician. It aims to provide initial protection within four months, after which maintenance treatment targets lasting protection.
Here is a video from the company’s website describing their program:https://player.vimeo.com/video/354062365
Click to visit sponsor
A phase 1 study of CA002 in 22 children aged 4 to 17 years in the UK showed good tolerance of peanut OIT. A subsequent phase 2 study of 99 children aged 7 to 16 years demonstrated the safety and efficacy of the oral immunotherapy candidate to treat peanut allergy, subject to confirmation in phase 3.
The phase 3 study is intended to confirm the efficacy and safety of CA002 in treating peanut allergy in patients aged 4 years and older. The company hopes to market the therapy for both children and adults in the US and Europe.
The description of CA002 resembles that of Palforzia, the only US FDA-approved food allergy treatment, also for peanut allergy. It remains to be seen if there is any appreciable difference between the two.Sources:
- Camallergy Awarded £1.1 M Funding Under Innovate UK Biomedical Catalyst Award for Novel Peanut Allergy Treatment — Company Press Release
- Clinical trials — Camallergy.com


Leave A Comment