;
FDA Announces New Draft Guidelines On Allergen Labeling
It’s been 16 years since Allergen Guidelines have be updated. The new draft
includes the existing allergens to now include sesame and three categories:
Fish (such as bass, flounder) Crustacean shellfish (such as crab, shrimp,
lobster) & Tree Nuts (such as almond, walnut, pecan). The new guidelines
also include protein and any other derivatives from the above allergens.
An example is the pea protein that is used flour, packaged and frozen foods)
Pea protein is a derivative of the peanut family!
FDA Issues Important New Draft Guidance on Allergen Labeling After 16 Years
-2022/12/01 4401

On Tuesday, the Food and Drug Administration (FDA) issued draft guidance that updates the previous edition of the Questions and Answers Regarding Food Allergens, Including the Food Allergen Labeling Requirements of the Federal Food, Drug, and Cosmetic Act.
This Fifth Edition updates the Q&A issued in 2006 by including sesame as the “Top 9” allergen (added by the FASTER Act) and answers questions related to allergen labeling for bulk foods, foods produced through genetic engineering, protein-free ingredients, and dietary supplements.
This information is of critical importance for all individuals concerned with food allergies, intolerances, and celiac disease. The Q&A answers many of the questions you may have regarding labeling.
Note that this is a draft. You may submit comments to the FDA by January 29, 2023, after which the FDA will issue the final document sometime after.
Here are a select number of questions and answers from the new draft. You can find the draft guidance in its entirety here.Click to visit sponsor
A. General Information
What foods and food groups are designated as “major food allergens”?
Foods: Milk, Eggs, Peanuts, Wheat, Soybeans, Sesame (effective 1/1/2023).
Food Groups: Fish (such as bass, flounder, cod), Crustacean shellfish (such as crab, lobster, shrimp), Tree nuts (such as almonds, walnuts, pecans)
B. Types of Foods That Fall Under the Food Allergen Labeling Requirements of the FD&C Act
Are food ingredients and finished foods subject to the food allergen labeling requirements of the FD&C Act?
Yes. Whether packaged in containers or in bulk for consumers or other food manufacturers,
food ingredients and finished foods (including those used to make dietary supplements, such as liquids, solids, powders, capsules, softgels, and tablets) that contain a major food allergen must comply with the allergen labeling requirements of section 403(w) of the FD&C Act. Food manufacturers, in particular, need to know if any major food allergens are present in the food ingredients they use to manufacture other products to ensure that ingredients containing major food allergens are properly handled and that finished product labels comply with the FD&C Act.
How should sesame be declared if it is used in a spice blend?
If sesame is used as an ingredient as part of a spice blend and is declared using the collective term “spice” or “spices” in accordance with 403(i) of the FD&C Act, such that its common or usual name does not appear in the ingredient list, it should be declared in a “Contains” statement or in a parenthetical after the collective term “spice” or “spices” in the ingredient list, i.e., “Contains sesame” or “spices (sesame).”
Are foods, including dietary supplements, derived from roots, leaves, stems, or bark of the same plant that bears tree nuts subject to the food allergen labeling requirements of the FD&C Act? What about wheatgrass or coconut sugar?
No. Roots, leaves, stems, bark, or other parts that are distinct from the tree nut portion of the plant are not major food allergens. For example, if a dietary supplement is derived from the leaves of the Ginkgo biloba L. plant, not the Ginkgo nut, and no other ingredients containing proteins derived from the Ginkgo nut or any other major food allergen were used to make the dietary supplement, the food allergen labeling requirements would not apply to the dietary supplement labeling. In the same way, the food allergen labeling requirements would not apply to wheatgrass or coconut sugar from coconut sap.
Which species of “fish” does FDA consider allergenic?
The FDA interprets “fish” as consisting of three categories:
- Jawless fish, such as hagfish and lampreys
- Bony fish, such as trout, flounder, bass, salmon, tilapia, cod, mackerel, tuna, and grouper
- Cartilaginous fish, such as shark, rays, and skates
Are pet foods or animal feeds subject to the food allergen labeling requirements of the FD&C Act?
No. FDA interprets section 403(w) of the FD&C Act to apply only to foods for human
consumption and to not apply to pet foods or animal feeds.
Are prescription or over-the-counter drugs, cosmetics, or household cleaning products subject to the food allergen labeling requirements of the FD&C Act?
No. FDA interprets section 403(w) of the FD&C Act to apply only to foods intended for human consumption and to not apply to prescription or over-the-counter drugs, cosmetics (such as face powder, body lotion, or bath soap), or household cleaning products (such as laundry detergent).
Are proteins from major food allergens, produced in other sources through the use of genetic engineering, subject to the food allergen labeling requirements of the FD&C Act?
Yes. The FD&C Act was intended to protect people with food allergies who could not determine what allergens were in their foods without labeling. Section 201(qq) of the FD&C Act (21 U.S.C. 321(qq)) defines a major food allergen as milk, egg, fish (e.g., bass, flounder, or cod), Crustacean shellfish (e.g., crab, lobster, or shrimp), tree nuts (e.g., almonds, pecans, or walnuts), wheat, peanuts, soybeans, and sesame effective January 20233 and also as a food ingredient that contains protein derived from these foods. Food ingredients that include proteins derived from a major food allergen (e.g., through chemical, biochemical, mechanical, fermentation, or bioengineering processes) may be capable of eliciting an allergic reaction and their presence is not obvious without declaration of the allergen. An example of such a product would be protein that is derived from cow milk but produced via fermentation in a non-milk food source, such as a genetically engineered strain of yeast. In this example, the protein produced by fermentation may be identical (or sufficiently similar) to the protein in milk such that it could be capable of binding to specific antibodies and causing allergic reactions to people sensitive to proteins in milk. Further, these proteins are “derived from” major food allergens because they are produced in a manner that uses the major food allergen’s DNA sequence. Therefore, we consider proteins from major food allergens, produced in other sources through the use of genetic engineering, to be derived from major food allergens and require allergen labeling under the FD&C Act. Such food ingredients should be declared in a way that makes the relationship to the source allergen clear. Some examples of how that could be achieved are: “Contains milk-derived protein” or “[ingredient name] (milk-derived protein).”Click to visit sponsor
C. Food Sources
For purposes of complying with the food allergen labeling requirements of the FD&C Act, what is “milk”?
For purposes of the definition of a “major food allergen” under section 201(qq) of the FD&C Act, FDA has historically interpreted “milk” as milk from the domesticated cow.
For purposes of complying with the food allergen labeling requirements of the FD&C Act, what are “eggs”?
For purposes of the definition of a “major food allergen” under section 201(qq) of the FD&C
Act, FDA has historically interpreted “eggs” as eggs from the domesticated chicken.
For the purpose of complying with the food allergen labeling requirements of the FD&C Act, what are tree nuts?
The FDA is aware that there is no universally accepted botanical definition of the term “tree nut.” Authoritative botanical references use many different botanical terms (e.g., berry, capsule, drupe, fruit, nut, and seed) to describe the embryo of a tree that can form into a dry, hard fruit considered to be a tree nut. Sometimes multiple, interchangeable botanical terms are used to describe the same tree nut. In addition to the tree nuts specifically cited in the definition of “major food allergen” in section 201(qq) of the FD&C Act (i.e., almonds, pecans, and walnuts) the table below lists examples of other tree nuts. We list tree nuts mentioned in the Senate Committee Report on FALCPA, 4 tree nuts identified by other federal agencies, tree nuts commonly marketed in the United States, and tree nuts that have been the subject of multiple questions to FDA. Each example is also recognized as a tree nut by one or more authoritative botanical references, and either the tree nut or its derivative (e.g., oil) has a documented history of use as a food for human consumption.
This list is not intended to be exhaustive because hundreds of types of tree nuts exist. Food
manufacturers are responsible for knowing the identity and content of all ingredients they use to make their products and whether the ingredients are or contain tree nuts or their proteins. Manufacturers are also responsible for declaring the specific type of nut as required by 21 U.S.C. 343(w)(2) and should use the common or usual name (or standardized common name as noted in Herbs of Commerce (21 CFR 101.4(h) for dietary supplements) listed below to declare the specific type of nut.
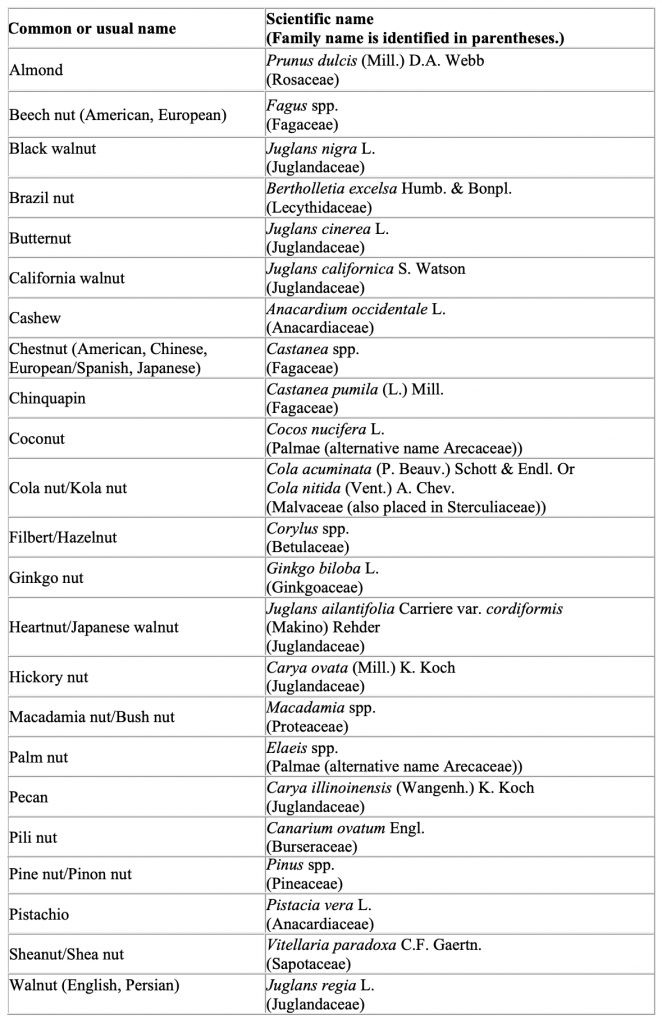
The scientific names for the tree nuts included in the table further clarify their identity. This
list uses broad scientific categories and uses the abbreviation “spp.” to indicate multiple species within a genus that represent tree nuts known by the corresponding common or usual name. However, the same genus may include other species that are not tree nuts and that currently have no human food use. Therefore, the fact that a particular species falls within a scientific category identified on this list does not mean that the species is appropriate for human food use.Click to visit sponsor
D. The Food Allergen Labeling Requirements of the FD&C Act
Where are the major food allergens required to be declared on the food label?
The food source of a major food allergen must be declared either in the ingredient list or in a “Contains” statement printed immediately after, or adjacent to, the ingredient list (section 403(w)(1) of the FD&C Act). If the food source is declared in the ingredient list and a “Contains” statement is also used, then the food source of all of the major food allergens present in the food also are to be declared in the “Contains” statement, even if they are declared in the ingredient list (section 403(w)(1) of the FD&C Act).

Under the food allergen labeling requirements of the FD&C Act, must individual units within a multiunit package have a “Contains” statement if each unit is not fully labeled?
The unit containers in a multiunit or multicomponent retail food package are exempt from
certain labeling requirements under 21 CFR 1.24(a)(14); however, such foods are not exempt from allergen labeling requirements, as section 403(w) of the FD&C Act is not listed as one of the exempted sections in that regulation. If the food is or contains a major food allergen, a “Contains” statement must be used (or the information can appear in the ingredient list if one is present). We suggest placing the allergen information near the statement of identity in the absence of an ingredient list.
However, no labeling is needed, including allergen labeling, if the individual unit is an
unlabeled inner sleeve intended solely for protection of the product, such as sleeves of crackers, and does not contain any written, printed, or graphic matter.
If an ingredient that contains a major food allergen is derived from several different species of the allergenic source, does each source need to be declared on the label?
Yes. If an ingredient is derived from several different species of an allergenic source, each
source must be declared on the label, e.g., “Fish gelatin (cod, haddock, pollock)” or
“Contains cod, haddock, pollock” (see section 403(w)(2) of the FD&C Act).
Is the name of the food source required to be declared in the ingredient list more than once if the food contains multiple ingredients that are or contain the same major food allergen?
No. The name of the food source is required to be declared only once in the ingredient
statement, even if the food contains multiple ingredients that are or contain that same major food allergen, unless the name of the food source that appears elsewhere in the ingredient list appears as part of the name of a food ingredient that is not a major food allergen (see section 403(w)(1)(B)(ii) of the FD&C Act). For example, both sodium caseinate and nonfat dry milk are derived from milk. If the ingredient list identifies sodium caseinate and nonfat dry milk as ingredients, the declaration of “milk” as part of the common or usual name “nonfat dry milk” would be sufficient.
Lactose is a milk sugar and ghee is a milk-derived fat. As a manufacturer, do I have to declare milk on the label if I use these ingredients?
The FD&C Act requires labeling of a food or ingredient that is or contains protein from a major food allergen (section 403(w) and 201(qq)). Ingredients derived from a major food allergen that do not contain proteins are not subject to FDA’s allergen labeling requirements and would not be subject to the labeling requirements described in section 403(w)(1) of the FD&C Act. If your major food allergen-derived ingredient is processed using technology that reliably produces a protein-free ingredient and you can ensure that the ingredient does not contain protein, then you would not have to declare the major food allergen on the label. While lactose is a milk sugar and ghee is a milk-derived fat, we understand that there is commonly residual protein from milk in these ingredients. When that is the case, lactose and ghee must be labeled in accordance with section 403(w)(1) of the FD&C Act. However, the manufacturer may consider the Food Allergen Labeling Exemptions Petition and Notification process if the firm believes that their ingredient qualifies for an exemption from section 403(w) of the FD&C Act.
When is a food ingredient derived from a major food allergen not subject to the food allergen labeling requirements of the FD&C Act?
Food ingredients, including flavors, colors, highly refined oils, and incidental additives, derived from a major food allergen are not subject to the food allergen labeling requirements of the FD&C Act when the food ingredients do not contain a protein from the major food allergen e.g., highly refined soybean oil. If the food ingredient contained such protein, then a petition or a notification would have to be submitted to FDA via the Food Allergen Labeling Exemptions Petition and Notification process in order to be exempted from the allergen labeling requirements of the FD&C Act.
As a manufacturer, how do I label a major food allergen that is also an incidental additive?
While you do not have to list incidental additives in the ingredient list (21 CFR 101.100(a)(3)), if an incidental additive contains a major food allergen, then you would have to declare the food source of the major food allergen (21 U.S.C. 343(w)(4)). For example, if you use wheat flour on the processing belt while processing rice crackers (as a processing aid), and do not declare “wheat” in the ingredient list, then you must list “wheat” in a “Contains” statement.
As a manufacturer, how do I label oils derived from a major food allergen?
The source of highly refined oils (“highly refined oils” are intended to signify refined, bleached, deodorized oils) that are derived from major food allergens are not required to be declared under section 403(w) of the FD&C Act. However, under the general ingredient labeling requirements, the source of all oils (whether highly refined or not) must be included as part of the common or usual name of the oil in the ingredient list on the label (such as soybean oil) (21 CFR 101.4(b)(14)). If a highly refined oil is exempt from ingredient labeling because it is an incidental additive in accordance with 21 CFR 101.100, there is no additional allergen labeling requirement in the FD&C Act.
If an oil that is derived from a major food allergen is not highly refined, then the source of the oil is required to be declared on the label in accordance with section 403(w) of the FD&C Act. Such labeling can be accomplished by either declaring the oil by its common or usual name in the ingredient list or declaring the source of the oil in a “Contains” statement, or both.
May a “Contains” statement be used to alert consumers to the presence of: (a) food allergens other than the major food allergens defined in the Act; and (b) food substances that are not food allergens to which individuals may be sensitive?
No. The purpose of the “Contains” statement as required by section 403(w) of the FD&C Act is to declare the presence of major food allergens. If the “Contains” statement was used to list: (a) food allergens other than the major food allergens; or (b) food substances that are not food allergens to which individuals may be sensitive, it may become more difficult for consumers to easily determine if a food contains a major food allergen, which would undermine the purpose of the “Contains” statement. Therefore, reserving the use of the “Contains” statement for its intended purpose enhances the clarity and directness of communicating information about the major food allergens for allergic consumers.
However, voluntary information that is truthful and not misleading may lawfully appear on the food label if it is consistent with other FDA labeling requirements. Thus, manufacturers could include voluntary information, such as the following, that is separate from the “Contains” statement that is required by section 403(w) of the FD&C Act:
- A manufacturer may alert consumers about the presence of a food allergen, other than a major food allergen, in a separate statement on the food label (such as “Other Allergen Information: mustard”).
- A manufacturer may alert consumers about the presence of a substance other than a food allergen to which they may be sensitive in a separate statement on the food label (such as “Other Information: Includes gluten”) or in the ingredient list.
Is a major food allergen that has been unintentionally incorporated in a food as the result of cross-contact subject to the food allergen labeling requirements of the FD&C Act?
No. The food allergen labeling requirements of the FD&C Act do not apply to a major food
allergen that is unintentionally incorporated in a food as a result of cross-contact. In the
context of food allergens, “cross-contact” occurs when an allergenic food is unintentionally
incorporated into another food that is not intended to contain that allergenic food. Cross-contact may result from many causes including customary methods of growing and harvesting crops, as well as from the use of shared storage, transportation, or production equipment.
However, allergen cross-contact is recognized as a potential hazard for consumers. FDA has
established requirements for food allergen preventive controls, as well as good manufacturing practices to protect food against food allergen cross-contact, in the Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food regulations in 21 CFR part 117. When these measures are not able to completely eliminate allergen cross-contact, some manufacturers have chosen to include allergen advisory statements to warn consumers about potential allergen cross-contact.Click to visit sponsor
E. Dietary Supplements
What types of dietary supplement ingredients are subject to the food allergen labeling requirements of the FD&C Act?
Section 403(w) of the FD&C Act applies to ingredients used to make dietary supplements that are or contain a major food allergen. This includes the following types of ingredients:
- Dietary ingredients – defined as, among other things, a vitamin, mineral, herb or other botanical, an amino acid, a dietary substance for use by man to supplement the diet by increasing the total dietary intake, or a concentrate, metabolite, constituent, extract, or combination of any such ingredients. Protein, if intended for use to supplement the diet by increasing the total dietary intake, is an example of a dietary ingredient (see section 201(ff) of the FD&C Act).
- Source ingredients – an ingredient that supplies the dietary ingredient. Sodium caseinate (derived from milk) is an example of a source ingredient for the dietary ingredient protein (see 21 CFR 101.36(d)).
- Other ingredients – examples of ingredients other than dietary ingredients or source ingredients include excipients, fillers, artificial colors, artificial sweeteners, flavors, or binders (21 CFR 101.4(g)).
When the major food allergen is already declared within the Supplement Facts label, must the allergen be declared elsewhere on the dietary supplement’s label?
No. We will consider the food allergen labeling requirements of the FD&C Act to be satisfied
when the major food allergen is declared within a Supplement Facts label. FDA regulations
(such as 21 CFR 101.36, 101.4(a)(1), and 101.4(g) and (h)) specify how dietary supplements must be labeled. When a source ingredient that supplies a dietary ingredient is identified in parentheses within the Supplement Facts label, or when the name of the dietary ingredient or its synonym is the source ingredient, it need not be repeated in the ingredient list that appears outside of the Supplement Facts label (see 21 CFR 101.36(d)). The complete listing of ingredients may be determined by consulting both the Supplement Facts label and any ingredient list on the dietary supplement label.
Must a separate “Contains” statement be provided on the label of a dietary supplement if all major food allergens are declared either within the Supplement Facts label or in an ingredient list?
No. A separate “Contains” statement is not needed if the major food allergens are declared
either within the Supplement Facts label or in an ingredient list. However, if a “Contains”
statement is used, the source of all of the major food allergens in the dietary supplement are to be included in the “Contains” statement.Source: Draft Guidance for Industry: Questions and Answers Regarding Food Allergen Labeling (Edition 5) — FDA


Leave A Comment